Advances in New Technology Add-On Payments for Innovative Medicine in China’s DRG/DIP Framework

In July, China’s National Healthcare Security Administration introduced the CHS-DRG Grouping Plan (Version 2.0), expanding the number of DRG groups from 376 to 634 to improve alignment with clinical standards. Alongside this enhancement, a core reform objective is to integrate the CHS-DRG technical framework with payment policies to address challenges related to adopting new medical technologies within the DRG/DIP payment model, particularly by easing the financial burden in the early stages of adoption.
As these changes unfold, our policy tracking and monitoring services have been actively following payment policy developments for innovative medical technologies across major risk-pooling regions. Here are some key highlights.
Section One: Evolving Regional Approaches to Reimbursing Innovative Medicines within the DRG/DIP Framework
Under China’s DRG/DIP system, reimbursement standards are generally defined by provincial healthcare security administrations or within specific BMI risk-pooling regions. Currently, regional reimbursement methods for innovative medical technologies fall into three main categories:
- Separate payments for all qualified new technologies or specific items
- Creation of new DRG/DIP groups or adjustments to existing group weights and rates
- Outlier fee-for-service payments for cases with high or low multipliers
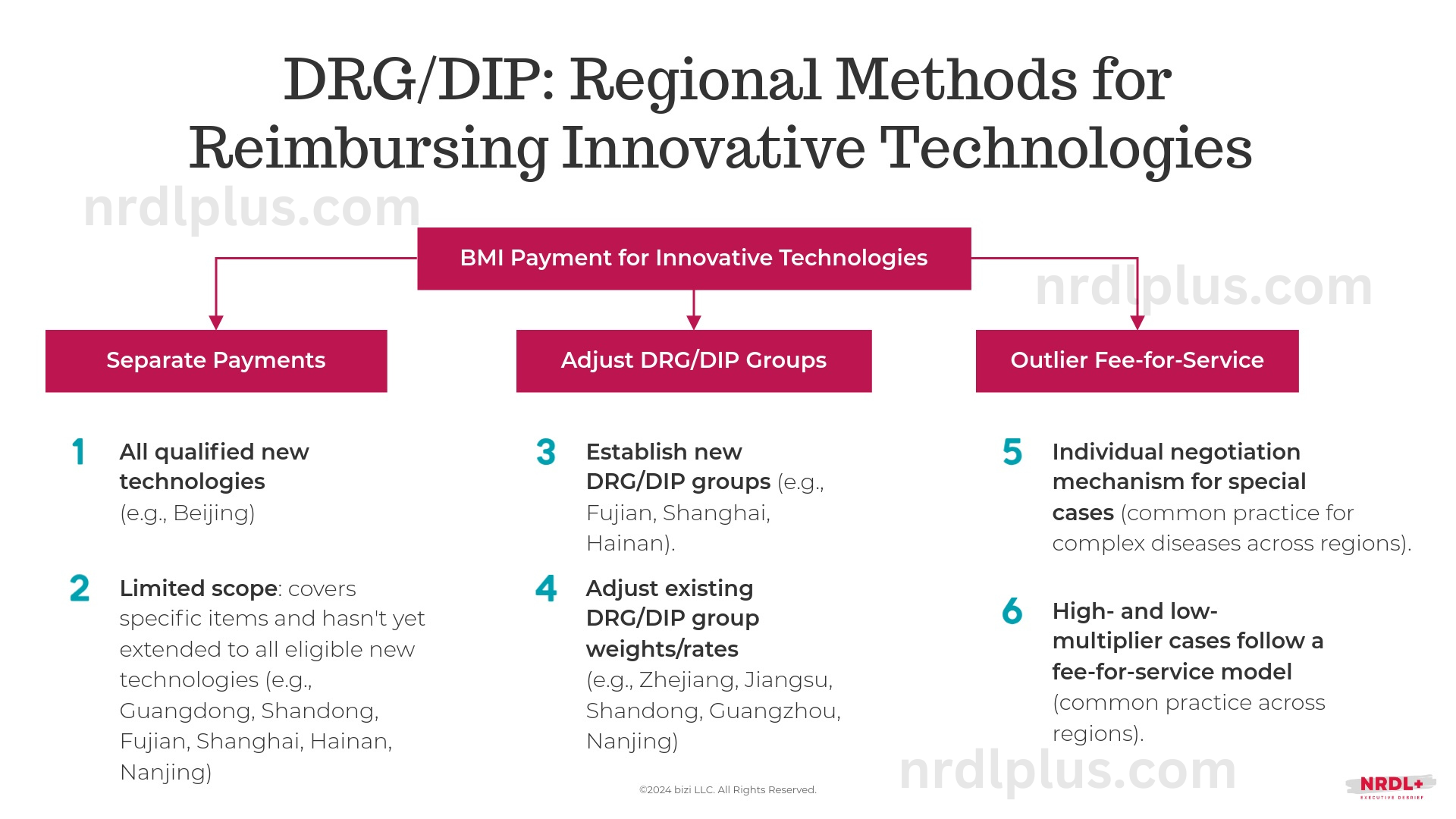
A region’s funding capacity is a significant factor in choosing the approach. For example, Beijing has implemented separate funding for all qualified new technologies, an approach more feasible for regions with ample insurance fund reserves and strong funding capabilities.
Section Two: Advances in Payment Policies for New Technology Add-On Payments
Eligibility Criteria
Beijing was among the first to implement separate payments for innovative medical technologies. On July 13, 2022, the Beijing Municipal Medical Security Bureau issued the Trial Measures for CHS-DRG Payment Management of New Drugs and Technologies, one of China's earliest policies outlining basic eligibility criteria for innovative technology add-on payments.
Zhejiang led as the first province to implement a DRG point-based payment model, granting additional points for qualified new technologies. In October, Zhejiang released the Management Measures for Innovative Medical Technology Insurance Payment Incentives (Trial), which further specifies the eligibility criteria for add-on payments for new technologies under China's DRG/DIP framework.
Aligned with the earlier policy from Beijing, Zhejiang’s criteria fall into five main categories:
- Newness: Eligibility for add-on payment is limited to technologies, services, or drugs within the defined newness period, beginning from NMPA approval, Basic Medical Insurance (MBI) listing, or new medical service price item approval.
- Cost: The average charge per case must surpass the jurisdictional threshold for add-on payment eligibility.
- Clinical Improvement and Innovation: The new technology must demonstrate marked clinical improvement over existing services.
- Case Volume: The technology must be used in a minimum number of annual cases involving basic medical insurance beneficiaries within the jurisdiction. This supports statistical stability in DRG groups, though both Beijing and Zhejiang waive this requirement for rare disease-related drugs and services.
- Medical Insurance Classification and Coding: The technology must be classified and coded by NHSA, facilitating brand and model tracking and integration with transaction, billing, and reimbursement systems in some provinces.
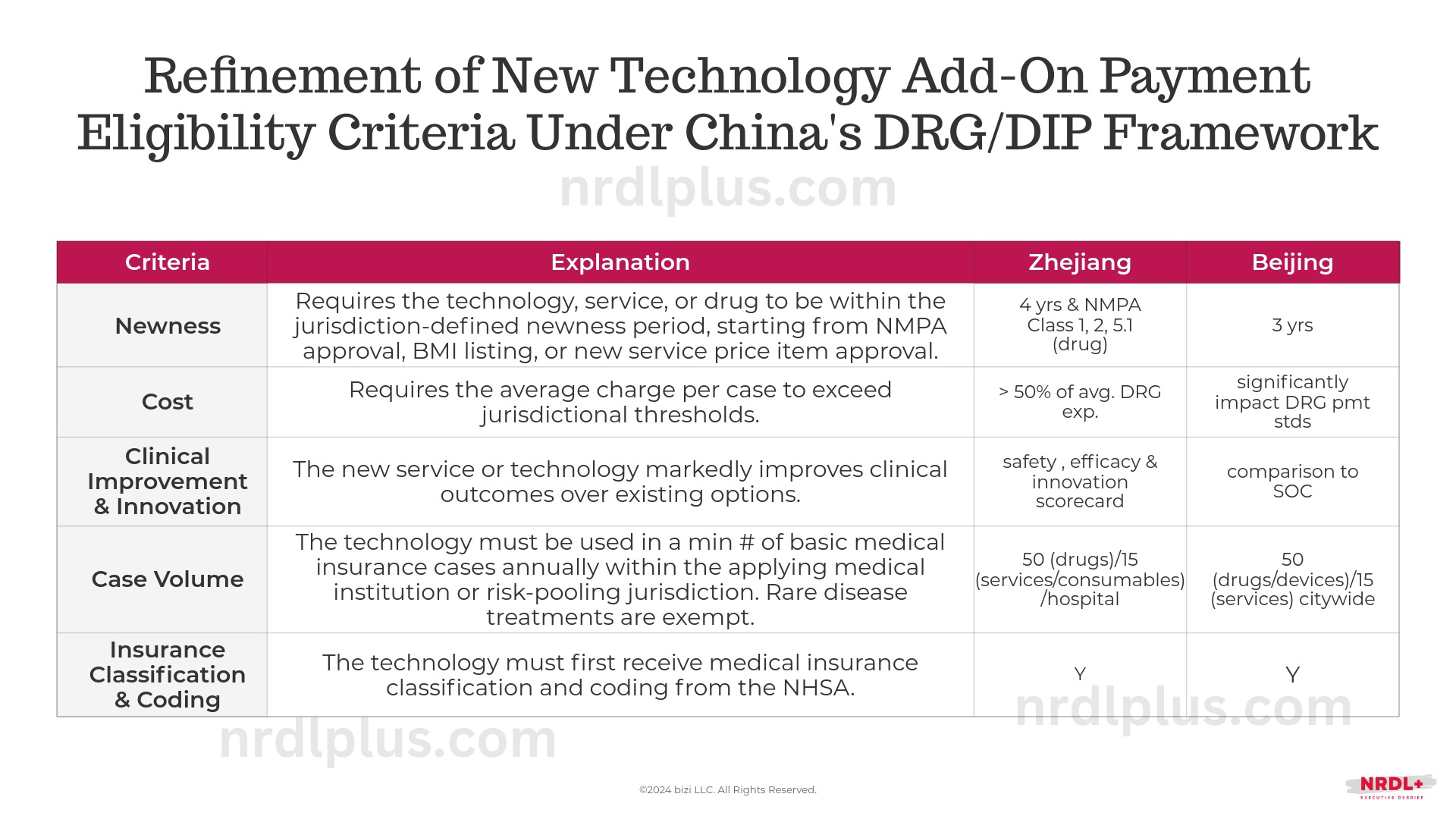
Zhejiang’s policy includes a specific cost threshold and a detailed scorecard assessing clinical improvement on safety, efficacy, and innovation, while Beijing primarily compares to the standard of care. Additionally, Beijing allows direct manufacturer applications, whereas Zhejiang requires applications from medical institutions.
Payment Standards
In Beijing, under the separate payment model, the Municipal Medical Insurance Center will settle the costs of exempt technologies annually based on actual usage over the next three years.
In Zhejiang, qualified new technologies receive additional points on top of the existing case grouping and payment structure. Each city-level healthcare security department can set the total incentive amount as a percentage of the annual DRG settlement. The incentive period typically spans three years, starting from the first implementation within each city and based on a full settlement year. Specifically,
(1) Incentives apply to cases meeting specific indications.
(2) For qualified high-multiplier cases, the trimmed portion is converted to points at 70%, then multiplied by the incentive coefficient to calculate additional incentive points. The incentive coefficient is 1 in the first year, 0.8 in the second, and 0.6 in the third, with extended de-escalation and higher coefficients for select cases (e.g., breakthrough innovations).
(3) For high-cost rare disease medications or medical services (including medical consumables) significantly affecting the DRG group’s average cost, payment is based on actual costs converted to points.
Assessments and Implementation
In Beijing, the scope of drugs, devices, and treatments exempt from CHS-DRG payment is set based on expert evaluations and data validation. Experts assess the clinical efficacy, innovation, and rationale of submitted items, while data validation analyzes their financial impact on the health insurance fund.
In Zhejiang, the Provincial Healthcare Security Bureau engages experts from various fields to assess the effectiveness, safety, innovation, cost impact, and applicability of innovative medical technologies using a scorecard:
(1) Effectiveness: Must show clear indications and significant treatment improvements (up to 20 points).
(2) Safety: Scored on expert consensus and inclusion in treatment guidelines (up to 20 points).
(3) Innovation: Evaluated on addressing unmet clinical needs and social benefit (up to 30 points).
(4) High Cost: Points awarded if case costs exceed 50% of the DRG group average over three years (up to 15 points).
(5) Application: Scored on provincial or regional clinical use, with case minimums of 50 for drugs and 15 for services (up to 15 points).
The expert results are then reviewed by the Bureau’s leadership, opened for public feedback, submitted for final approval by the Bureau’s Director, and published for implementation.
To date, neither Beijing nor Zhejiang has established a detailed public timeline for submitting, reviewing, and approving new technology add-on payment requests.
Effective policy implementation will also require coordination with the Health Commission, which oversees China’s medical institutions, to establish clear guidelines on key hospital metrics, such as drug and device proportion control and physician evaluations.
References:
- 医保付费新纪元:DRG2.0版分组逻辑与升级亮点解析, 中国医疗保险, 2024-08-08, http://www.phirda.com/artilce_35993.html?cId=4&module=trackingCodeGenerator
- 北京市医疗保障局发布《关于印发CHS-DRG付费新药新技术除外支付管理办法的通知(试行)》(京医保中心发〔2022〕30号), 2022-7-13, https://ybj.beijing.gov.cn/tzgg2022/202207/t20220713_2798069.html
- 《浙江省医疗保障局关于印发浙江省创新医药技术医保支付激励管理办法(试行)的通知》 (浙医保发〔2024〕17号), 2024-10-12, http://ybj.zj.gov.cn/art/2024/10/12/art_1229113757_2530959.html





